
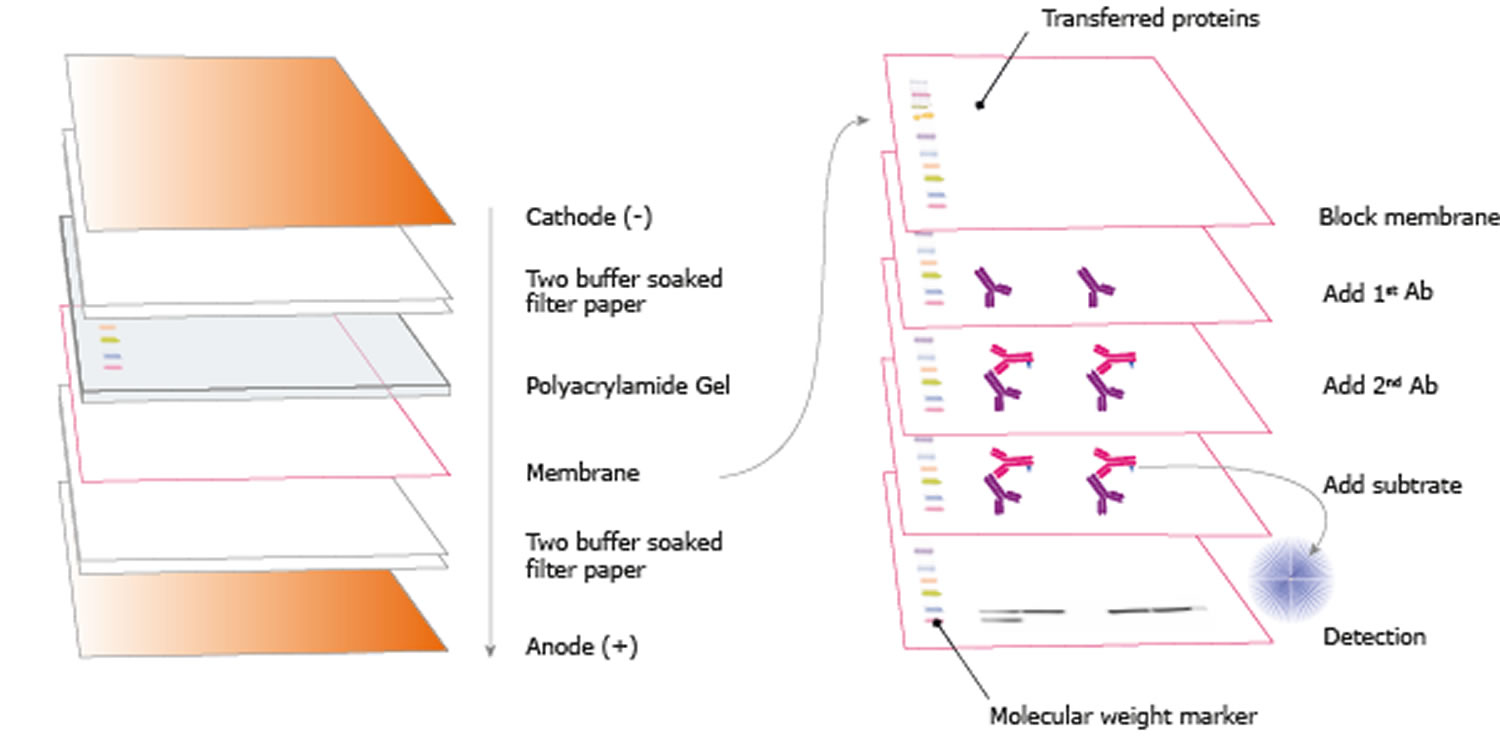
Each system provides unique advantages when resolving proteins of different molecular weights.Ĭontinue reading: Overview of Protein ElectrophoresisĮxplore: Protein Gel electrophoresis productsįollowing electrophoresis, the protein must be transferred from the gel to a membrane. Several buffering systems or gel chemistries are available for protein gel electrophoresis. In contrast, sodium dodecyl sulfate-PAGE, or SDS-PAGE, separates proteins according to mass due to the negative charge imparted on proteins bound to the ionic SDS detergent. For example, nondenaturing PAGE, or native PAGE, separates proteins according to their mass-charge ratios. Several forms of PAGE exist and can offer different types of information about the protein(s) of interest. When combined with western blotting, PAGE is a powerful analytical tool providing information on the mass, charge, purity or presence of a protein. Proteins are commonly separated using polyacrylamide gel electrophoresis (PAGE) to characterize individual proteins in a complex sample or to examine multiple proteins within a single sample. Gel electrophoresis is a technique in which charged molecules, such as protein or DNA, are separated according to physical properties as they are forced through a gel by an electrical current. The indirect method offers many advantages over the direct method, which are described below. Labels (or conjugated molecules) may include biotin, fluorescent probes such as Invitrogen Alexa Flour or DyLight fluorophores, and enzyme conjugates such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). Subsequently, the primary antibody is detected using an enzyme- or fluororophore-conjugated secondary antibody. In the indirect detection method, an unlabeled primary antibody is first used to bind to the antigen. This detection method is not widely used as most researchers prefer the indirect detection method for a variety of reasons. With the direct detection method, an enzyme- or fluorophore-conjugated primary antibody is used to detect the antigen of interest on the blot. One common variation involves direct versus indirect detection. Procedures vary widely for the detection step of a western blot experiment. Whatever system is used, the intensity of the signal should correlate with the abundance of the antigen on the membrane. Fluorescent blotting is a newer technique and is growing in popularity as it affords the potential to multiplex (detect multiple proteins on a single blot). Alternatively, fluorescently tagged antibodies can be used, which require detection using an instrument capable of capturing the fluorescent signal. However, digital imaging instruments based on charge-coupled device (CCD) cameras are becoming popular alternatives to film for capturing chemiluminescent signal. The light output can be captured using film. The most sensitive detection methods use a chemiluminescent substrate that produces light as a byproduct of the reaction with the enzyme conjugated to the antibody. Chromogenic substrates produce a precipitate on the membrane resulting in colorimetric changes visible to the eye. Often the secondary antibody is complexed with an enzyme, which when combined with an appropriate substrate, will produce a detectable signal. Most commonly, the transferred protein is then probed with a combination of antibodies: one antibody specific to the protein of interest (primary antibody) and another antibody specific to the host species of the primary antibody (secondary antibody). Next, the membrane is blocked to prevent any nonspecific binding of antibodies to the surface of the membrane. Subsequently, the separated molecules are transferred or blotted onto a second matrix, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane. The first step in a western blotting procedure is to separate the macromolecules in a sample using gel electrophoresis.


 0 kommentar(er)
0 kommentar(er)
